Extrasystoles ventriculaires
isolées, répétitives ou organisées en tachycardie ventriculaire.
Le potentiel évolutif vers une arythmie ventriculaire sévère, et donc le risque de mort subite, repose sur :
Des critères ECG
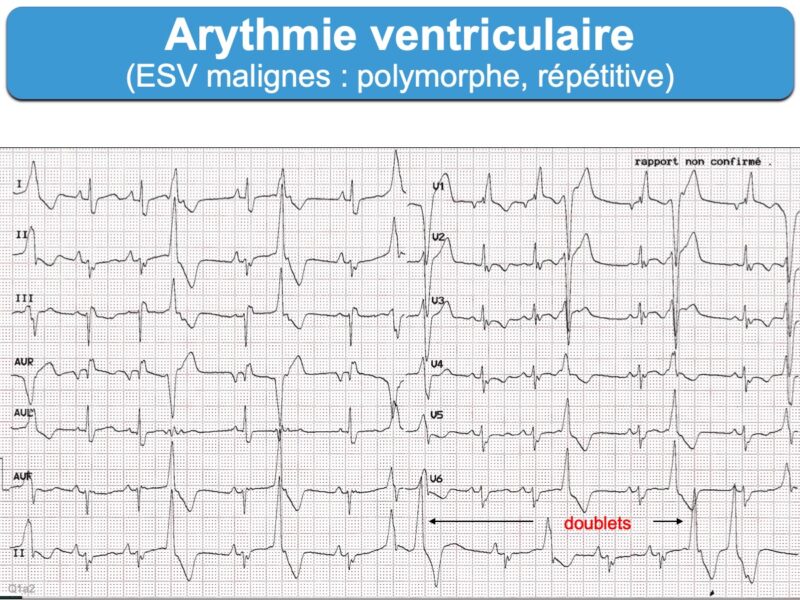
– Extrasystoles ventriculaires : avec retard droit ou retard gauche, monomorphe ou polymorphe, isolées ou fréquentes (> 30/h), répétitive (doublets ou salves), à couplage long ou court (Cf. Extrasystoles ventriculaires malignes [2])
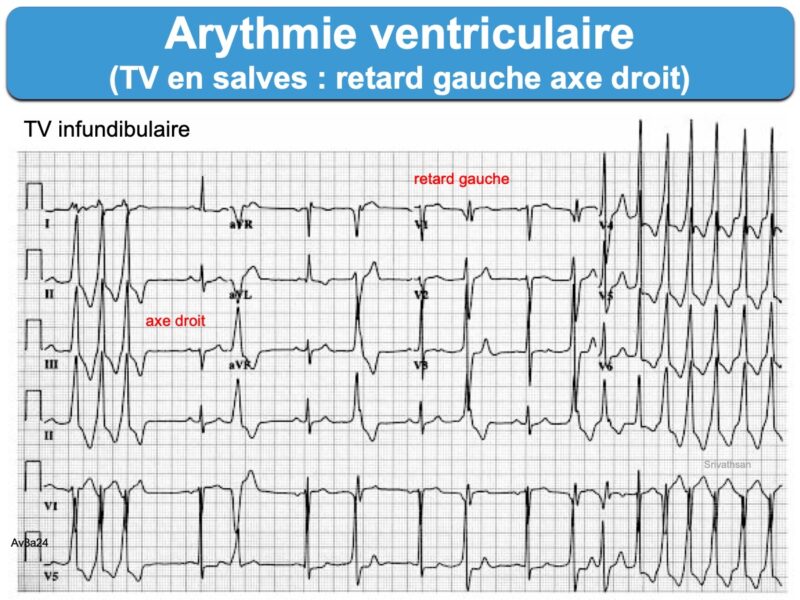
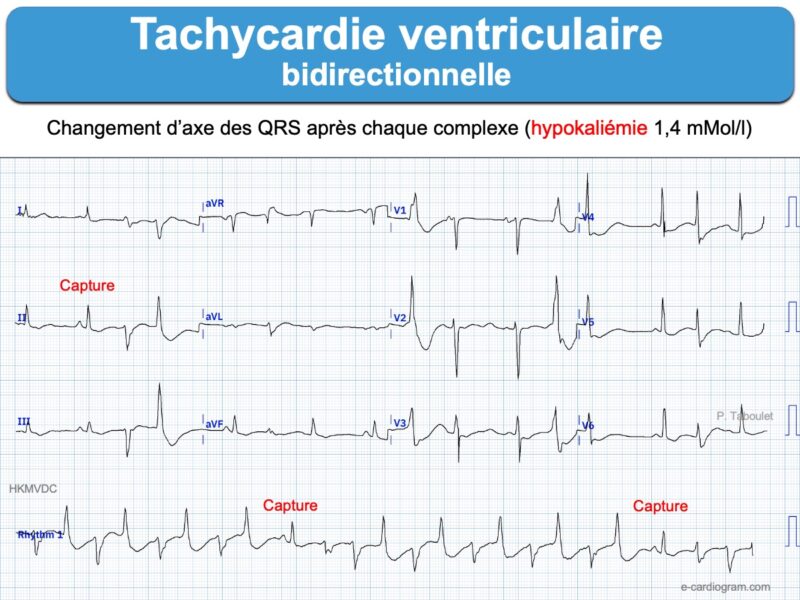
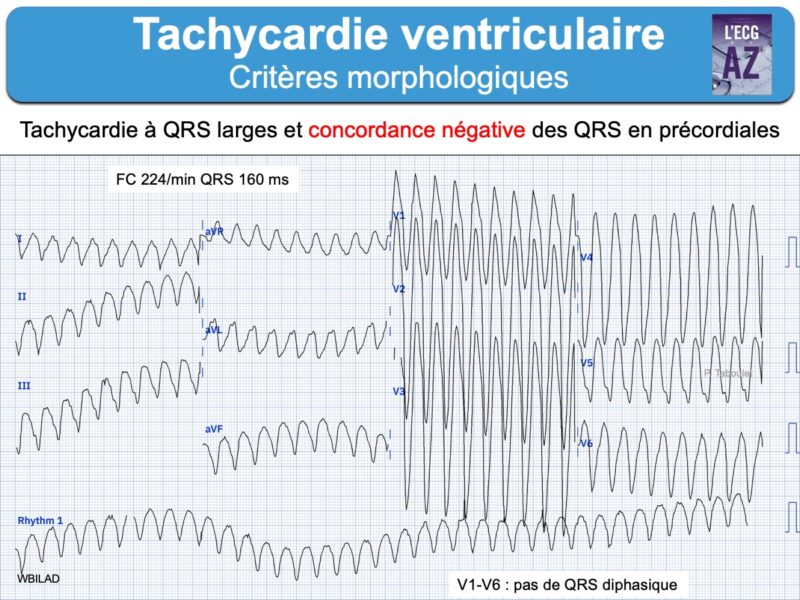
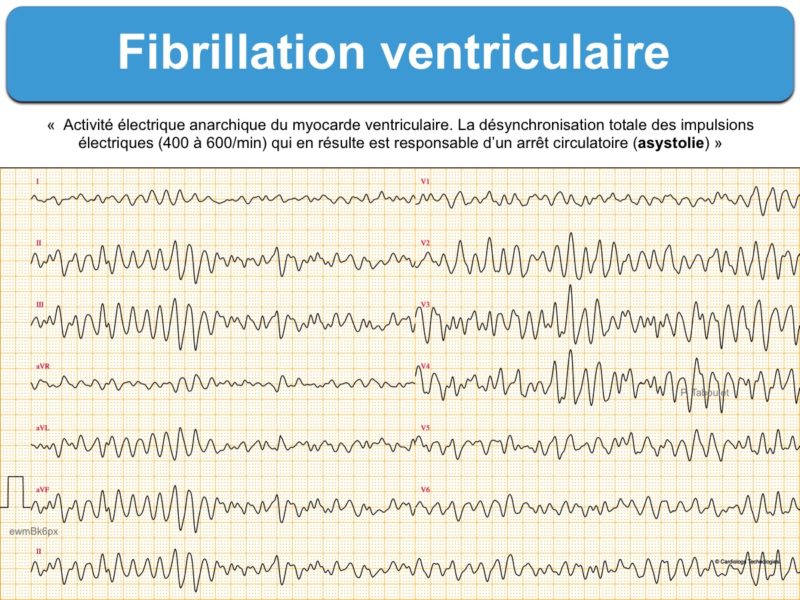
– Tachycardie ventriculaire : salve > 3 ESV de fréquence > 100/min, non soutenue ou soutenue (> 30 sec) « commune » ou « spécifique » (TV fasciculaire, TV infundibulaire, TV catécholergique, TV bidirectionnelle, torsade de pointes, orage rythmique, flutter ventriculaire ou fibrillation ventriculaire).
Des critères cliniques
- mode déclenchement : au repos, à l’effort, après l’effort
- symptômes : asymptomatique, symptômes minimes, pré-syncope, syncope, arrêt cardiaque (récupéré) ou mort subite
Le terrain cardiologique
- cœur structurellement normal
- cardiopathie ischémique, cardiomyopathie, cardiopathie congénitale…
- canalopathie (ex. Syndrome de Brugada, syndrome du QT long…)
- existence d’une anomalie métabolique ou toxique (Cf. ECG toxique, ECG métabolique)
Le mécanisme
Merci de me contacter si vous souhaitez améliorer ce contenu.

Lire aussi : Extrasystoles ventriculaires bénignes, Extrasystoles ventriculaires malignes, Défibrillateur, Cardioversion
Mise au point dispo en ligne (très complète) rev med suisse
Vidéos YouTube. P. Taboulet
- 10a. De l’extrasystole droite à la TV.
- 10b. De l’ESV gauche à la tachycardie ventriculaire
- 10c. Arythmie ventriculaire, Brugada, QT long
- 10d. Prise en charge d’une tachycardie à complexes QRS larges (VT score)
- Quiz tachycardie 165 bpm avec QRS larges
[1] Binah O, Rosen MR. Mechanisms of ventricular arrhythmias. Circulation 1992; 85(1 Suppl):I25-31. Review. (pas d’accès on line) Ventricular arrhythmias may result from abnormalities of impulse initiation and/or impulse propagation. The former include automatic arrhythmias, which may occur at high (normal) levels of membrane potential or at low (abnormal) levels of membrane potential. They also include triggered activity, which may result from early (occurring before complete repolarization) or delayed (occurring after complete repolarization) afterdepolarizations. Arrhythmias resulting from abnormal impulse propagation may be reentrant, determined in part by anatomic or functional conduction block, or the result of reflection. The factors determining various arrhythmogenic mechanisms are discussed.
[2] Lown B, Wolf M. Approaches to sudden death from coronary heart disease. Circulation. 1971 Jul;44(1):130-42. (téléchargeable)